Betaferon
Generic Name: interferon beta-1b (rbe)
Product Name: Betaferon
Indication: What Betaferon is used for
Single clinical event indicating high risk of developing MS: Betaferon is for use in patients who for the first time have experienced symptoms which indicate a high risk for development of multiple sclerosis. Your doctor will rule out any other reasons which could explain these symptoms before you are treated.
Relapsing-remitting MS: Betaferon is for use in patients who have occasional attacks or relapses during which symptoms become noticeably worse (at least two relapses within the last two years). This is the form of multiple sclerosis where patients experience attacks of CNS problems (for example of visual disturbances, arm/leg weakness, loss of bowel/bladder control) separated by periods of complete or incomplete recovery.
Betaferon has been shown to cut down the number of attacks and make them less severe. It reduces the number of hospital stays due to the disease and prolongs the time without relapses.
Secondary progressive MS: Betaferon is also for use in patients whose symptoms progress to another form of MS called secondary progressive MS. With this, patients become increasingly impaired, whether or not they have relapses. Betaferon can reduce the number and severity of the attacks, and slow the progression of disability.
Betaferon has been shown to have a marked effect on those physical changes in the brain that can be detected by a special kind of brain scan. Ask your doctor if you have any questions about why Betaferon has been prescribed for you.
This medicine is available only with a doctor’s prescription.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
Action: How Betaferon works
Betaferon belongs to a class of medicines known as interferons. Interferons are naturally occurring proteins produced by the body that help fight against attacks on the immune system such as viral infections.
The active substance of Betaferon is interferon beta-1b, a recombinant human interferon beta produced from a strain of Escherichia coli.
Multiple sclerosis (MS) is a long-term condition that affects the central nervous system (CNS) i.e. brain and spinal cord. The exact cause of MS is unknown. An abnormal response by the body’s immune system is thought to play an important part in the process which damages the CNS.
Betaferon has been shown to change the response of the immune system and to help reduce disease activity.
Betaferon contains 0.25 mg of the active ingredient interferon beta-1b (rbe) per mL.
It also contains the inactive ingredients mannitol, human albumin, and 0.54 % sodium chloride solution (as diluent).
The inactive ingredients of Betaferon include small amounts of mannitol (a naturally occurring sugar) and human albumin (a protein). If you know that you are hypersensitive to any of the ingredients or if you become so, you must not use Betaferon.
Dose advice: How to use Betaferon
Before you use Betaferon
When you must not use it
Do not use Betaferon if you have an allergy to:
- Any medicine containing interferon beta-1b (rbe), mannitol or human albumin;
- Any of the ingredients listed here.
Some of the symptoms of an allergic reaction may include:
- Shortness of breath;
- Wheezing or difficulty breathing;
- Swelling of the face, lips, tongue or other parts of the body;
- Rash, itching or hives on the skin.
Do not use this medicine if you are pregnant. It may affect your developing baby if you take it during pregnancy.
Do not use this medicine if you have liver failure.
Do not use this medicine if you suffer from epilepsy (fits or seizures) which is not controlled by other medicines.
Do not use this medicine after the expiry date printed on the pack and blister. The expiry date is printed on the carton and on each blister after “EXP” (e.g. 11 2018 refers to November 2018). The expiry date refers to the last day of that month. If it has expired return it to your pharmacist for disposal.
Do not use this medicine if the packaging is torn or shows signs of tampering. If the packaging is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start using this medicine, talk to your doctor.
Before you start to use it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- Seizures (fits or convulsions);
- Severe depression (feeling of severe sadness and unworthiness) or thoughts of suicide;
- Heart disorders;
- Kidney disease;
- Liver disease;
- Blood disorder (e.g. low counts of platelets, red and white blood cells);
- Thyroid disease;
- Pancreatitis;
- An increase of certain type of blood fats (triglycerides);
- Allergy to any other medicines, foods, dyes or preservatives;
- Bone marrow disorder;
- Monoclonal gammopathy ( a disorder of immune system where abnormal proteins are found in the blood).
Tell your doctor if you are pregnant or plan to become pregnant or are breastfeeding. It is not known whether Betaferon passes into breast milk. If you want to breastfeed while using Betaferon, discuss this with your doctor. Your doctor can discuss with you the risks and benefits involved.
Women of childbearing age should take appropriate contraceptive measures while using Betaferon. If you become pregnant while using Betaferon, stop your treatment and contact your doctor.
If you have not told your doctor about any of the above, tell them before you start taking Betaferon.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop. Care must be taken when Betaferon is used with some medicines, including some used to treat fever and pain.
The following medicines which are commonly used by people with MS are safe to take while using Betaferon:
- Corticosteroids such as hydrocortisone, prednisone or prednisolone;
- ACTH (adrenocorticotrophic hormone).
Some medicines that are broken down by the liver and Betaferon may interfere with each other. These include:
- Medicine to treat epilepsy;
- Medicine used for sedation or to treat anxiety;
- Medicine to treat depression.
Your doctor may have to adjust the dose of your other medicines while you are using Betaferon. It is therefore important that you tell your doctor about all the medicines that you are taking.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
What else you should know
- This product contains human albumin and hence carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jacob Disease (CJD) is also considered extremely remote;
- It is not known if Betaferon can decrease your ability to have children in the future;
- During treatment with Betaferon, your body may produce substances called neutralising antibodies, which may react with Betaferon. It is not yet clear if these reduce the effectiveness of the treatment. Neutralising antibodies are not produced in all patients.
Betaferon is not recommended for use in children under 18 years of age with MS as there is no clinical experience in this age group.
How Betaferon is used
If you do not understand the instructions provided, ask your doctor, MS education nurse or pharmacist for help.
Follow all directions given to you carefully. They may differ from the information contained here.
Betaferon is given as a subcutaneous (under the skin) injection after reconstitution every other day.
Treatment with Betaferon should be started under the supervision of a specialist doctor experienced in the treatment of MS. It is recommended that new users of Betaferon contact the MS immunotherapy nurse to assist with training. A list of contact numbers is included under Further Information. The service is available at no cost.
Before administration, the Betaferon solution for injection has to be prepared from a vial of Betaferon and the 1.2 mL of diluent from the pre-filled syringe.
Each mL of reconstituted solution for injection contains 8 million IU (international units) or 0.25 mg interferon beta-1b (rbe).
The usual dose is 1 mL of the prepared Betaferon solution for injection is injected subcutaneously (under the skin) every other day.
Betaferon should not be injected directly into the veins.
The instructions included here are for manual self-injection. Auto-injectors are also available for use with Betaferon. If an auto-injector is used, please follow instructions enclosed with your auto-injector.
How much is given
In general, treatment should be started at a low dose of 0.25 mL (0.0625 mg). The dose will then be increased gradually to the full dose of 1.0 mL (0.25 mg) which is equivalent to 8 million IU.
The dose should be increased at every fourth injection in four steps (0.25 mL, 0.5 mL, 0.75 mL, 1.0 mL). Your doctor may decide together with you to change the time interval for the dose increase depending on side effects you may experience at the start of treatment.
Administration will either be done by your doctor or their assistant or by yourself after you have been carefully and sufficiently instructed and trained. To assist you in subcutaneous self-administration of Betaferon, detailed instructions for self-injection are set out below. These instructions also tell you how to prepare the Betaferon solution for injection.
To reduce the risk of the injection solution becoming contaminated it should be used as soon as possible after it is prepared. If required, the reconstituted solution for injection may be stored in the refrigerator (not freezer) at 2°C to 8°C and used within 3 hours of preparation.
How to inject
Betaferon is intended to be injected by yourself by subcutaneous (under the skin) injection. The following instructions and pictures explain how to prepare Betaferon for injection and how to inject Betaferon yourself.
Please read the instructions carefully and follow them step by step. Your doctor or their assistant will help you to learn the process of self-administration. Do not attempt to inject yourself until you are sure that you understand how to prepare the injection solution and give the injection to yourself.
The instructions include the following main steps:
- General advice;
- Getting ready to inject;
- Reconstituting the solution, step by step;
- Drawing up the injection;
- Giving the injection;
- A quick review of the process.
General advice
- Be consistent, use Betaferon as described under “How Betaferon is used”;
- Keep your syringes and syringe disposal unit out of reach of children; lock the supplies away if possible;
- Never re-use syringes or needles;
- Always use a sterile (aseptic) technique as described here. If in any doubt, discard needles, syringes or solution and start again;
- Always place the used syringes in the proper disposal unit.
Getting ready to inject
Choosing an injection site
Before preparing your injection, decide where you are going to inject. You should inject Betaferon into the fatty layer between the skin and muscle (that is, subcutaneously, about 8 to 12 mm under the skin). The best places for injections are where the skin is loose and soft, and away from joints, nerves, or bones, for example, the abdomen, arm, thigh, or buttocks. Try to avoid the panty or belt line at the waist and the seat portion of the buttocks as daily activity may irritate these areas.
Important: Do not use any area where you can feel lumps, bumps, firm knots, pain or an area that is discoloured, indented, scabbed, or where skin is broken. Talk to your doctor or healthcare professional about these or any other unusual conditions you may find.
You should rotate the injection site at every injection. If some areas are too difficult for you to reach, you may need to ask your support person (or someone who has been trained to give injections) to help you with these injections. Follow the sequence described in the schedule here (see “Rotating injection sites”) and you will come back to your first injection site area after 8 injections (16 days). This will give each injection site a chance to fully recover before receiving another injection.
Please refer to the rotation schedule here to learn how to choose an injection site. An example of a medication record is also included. This should give you an idea of how you can keep track of your injection sites and dates.
Checking the content of the pack
You will find in the Betaferon pack:
- 1 Betaferon vial (with powder for solution for injection);
- 1 pre-filled syringe of diluent for Betaferon (sodium chloride solution 5.4 mg/mL (0.54%)). (Be sure that the tip cap is firmly attached to the solvent syringe.);
- 1 vial adapter with a pre-attached needle;
- 2 alcohol swabs for skin and vial cleaning.
In addition, you will need a disposal unit for used syringes and needles. For skin disinfection use an appropriate disinfectant.
Reconstituting the solution, step by step
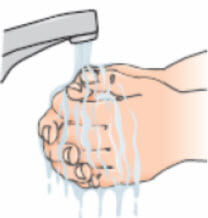
- Wash your hands thoroughly with soap and water before beginning this process;
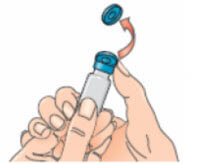
- Open the Betaferon vial and put it on the table. It is best to use your thumb rather than your nail as it could break;
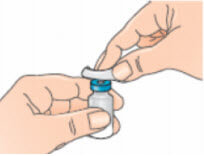
- Clean the top of the vial with an alcohol wipe, moving the wipe in one direction only. Leave the wipe on top of the vial;
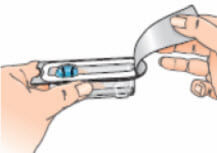
- Open the blister pack containing the vial adapter, but leave the vial adapter inside. Do not remove the vial adapter from the blister pack at this stage. Do not touch the vial adapter. This is to keep it sterile;
- Rest the vial on a flat surface while attaching the adapter;
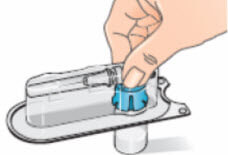
- Remove the alcohol wipe from the top of the Betaferon vial. Place the blister pack containing the vial adapter on top of the vial. Push it down with your thumb and forefinger or the palm of your hand until you feel it snap into place;
- Remove the blister pack from the vial adapter, holding the blister edges. Now you are ready to attach the pre-filled diluent syringe to the vial adapter;
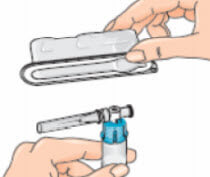
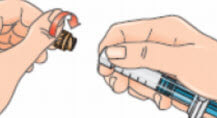
- Pick up the syringe. Remove the orange tip cap, using a ‘twist-and-pull’ motion. Throw away the tip cap;
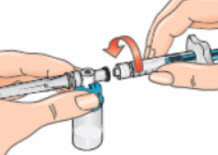
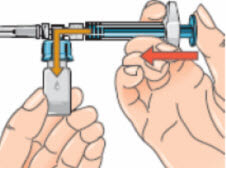
- Connect the syringe to the opening on the side of the vial adapter by inserting the end of the syringe and tightening carefully with a clockwise “push-and-twist” motion (see arrow). This will form the syringe assembly;
- Hold the syringe assembly at the bottom of the vial. Slowly push the plunger of the syringe in all the way to transfer all of the diluents into the vial. Release the plunger. The plunger may go back to its original position;
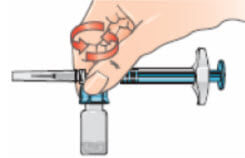
- With the syringe assembly still attached, swirl the vial around gently to completely dissolve the dry Betaferon powder;
- Do not shake the vial;
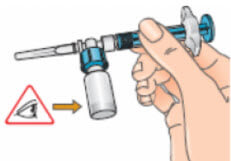
- Examine the solution carefully. It should be clear and contain no particles. If the solution is discoloured or contains particles, discard it and start again with a new single pack of supplies. If foam is present – which can happen if the vial is shaken or swirled too much – let the vial sit undisturbed until the foam settles;
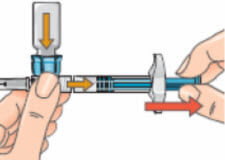
- If the plunger has moved back to its original position push it in again and hold it in place. To prepare your injection, turn the assembly over so that the vial is on top, cap side pointing down. Doing this allows the solution to flow down into the syringe.
Drawing up the injection
Keep the syringe horizontal. Slowly pull the plunger back to withdraw all the solution out of the vial and into the syringe.
- Turn the syringe assembly so that the needle is pointing up. This allows any air bubbles to rise to the top of the solution;
- Remove any air bubbles by gently tapping the syringe and pushing the plunger to the 1ml mark, or to the volume prescribed by your doctor;
- If too much solution goes into the vial along with the air bubbles, pull the plunger back a little to withdraw the solution back from the vial into the syringe. Do this until all the air is gone and there is 1 ml of reconstituted solution in the syringe;
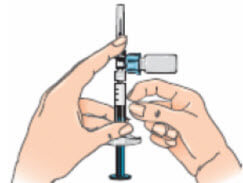
- Important: You will need to hold the syringe assembly to a horizontal position with the vial on top when withdrawing solution again;
- Next, hold the blue vial adapter with the attached vial and remove it from the syringe by twisting it and then pulling it down, away from the syringe;
- Only hold the blue plastic adapter when removing. Keep the syringe in a horizontal position and the vial below the syringe;
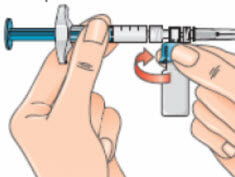
- Removing the vial and adapter from the syringe (using the twist and pull motion) ensures that the solution will flow out from the needle when injected;
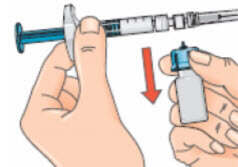
- Dispose of the vial and any unused portion of the solution in the disposal unit.
You are now ready to inject.
If, for some reason, you are not able to inject the Betaferon immediately, you can keep the reconstituted solution in the syringe in a refrigerator for up to 3 hours before using. Do not freeze the solution, and do not wait longer than 3 hours to inject it. If more than 3 hours pass, discard the reconstituted Betaferon solution and prepare a new injection. When you use the solution, warm it up in your hands before injecting to avoid pain.
Giving the injection subcutaneously (under the skin)
- Choose an area for the injection (see advice at the start and diagrams here), and make a note of it in your medication record;
- Use an alcohol swab to clean the skin at the injection site. Let the skin air-dry. Throw the swab away. For skin disinfection use an appropriate disinfectant;
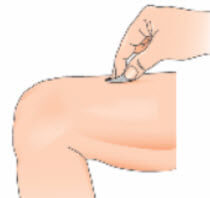
- Remove the cap from the needle. Pull the cap, do not twist it;
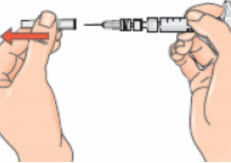
- Gently pinch the skin together around the disinfected injection site (to raise it up a little).
Please follow manufacturer’s instructions for use with an autoinjector. Alternatively, for manual injection:
- Holding the syringe like a pencil or a dart, push the needle straight into the skin at a 90º angle with a quick, firm motion;
- Inject the medicine using a slow, steady push on the plunger. (Push the plunger all the way in until the syringe is empty.);
- Discard the syringe in the disposal unit.
Quick review of the process
- Take out the contents of the pack;
- Attach vial adapter to the vial;
- Connect the syringe to the vial adapter;
- Push syringe plunger to transfer diluent into the vial;
- Turn the syringe assembly over, then pull out the plunger;
- Remove vial from syringe –– you are now ready to inject.
Note: The injection should be administered immediately after mixing (if the injection is delayed, refrigerate the solution and inject it within 3 hours). Do not freeze.
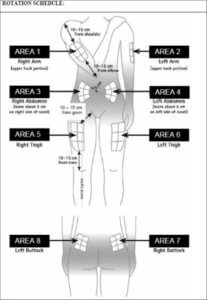
Rotating injection sites
You need to choose a new site for each injection to allow the area time to recover and help prevent infection. Advice on which areas to choose is given here. It is a good idea to know where you plan to inject before you prepare your syringe. The schedule shown below will help you to vary the sites appropriately. For example, give the first injection into the right side of the abdomen, choose the left side for the second injection, then move to the right thigh for the third, and so on through the diagram until all suitable areas of the body have been used. Keep a record of where and when you last gave yourself an injection. One way to do that is to note the injection site on the enclosed medication record card.
By following this schedule, you will come back to your first area (e.g. the right side of the abdomen) after 8 injections (16 days). This is called a Rotation Cycle. On our example schedule each area is split again into 6 injection sites (which adds up to 48 injection sites altogether), left, right, upper, middle and lower part of each area. If you come back to an area after one Rotation Cycle, choose the most distant injection site within this area. If an area becomes sore, talk to your doctor or nurse about choosing other injection sites.
Rotation schedule
To help you rotate the injection sites appropriately we recommend that you keep a record of the date and location of your injection. You can use the following rotation schedule.
Work through each rotation cycle in turn. Each cycle will be 8 injections (16 days), given in area 1 through to area 8 in turn. By following this sequence, you will give each area a chance to recover before receiving another injection.
Rotation Cycle 1: Upper left section of each area.
Rotation Cycle 2: Lower right section of each area.
Rotation Cycle 3: Middle left section of each area.
Rotation Cycle 4: Upper right section of each area.
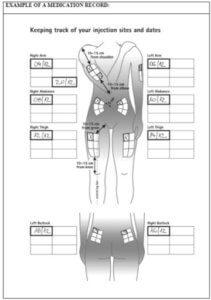
Betaferon medication record
Instructions for keeping track of your injection sites and dates:
- Start with your first injection (or your last injection if you are not a new Betaferon user);
- Select an injection site. If you have already been using Betaferon, start with the area that has not been used during the last rotation cycle, i.e. the past 16 weeks.);
- After your injection, fill in the used injection site and date on the table in your injection record (see the example: Keeping track of your injection sites and dates).
How long to take it
The length of treatment will be decided by you and your doctor.
If you forget to use it
If you have forgotten to give yourself an injection at the right time, do it as soon as you remember and then follow on with the next one 48 hours later. Do not take a double dose to make up for the forgotten single dose.
Ask your doctor if you are not sure what to do or have trouble remembering to inject your medicine.
If you stop using Betaferon
Talk to your doctor if you stop or wish to stop treatment. Stopping Betaferon is not known to lead to acute withdrawal symptoms.
If you inject too much (overdose)
Administration of many times the dose of Betaferon recommended for treatment of multiple sclerosis has not led to life-threatening situations. In case of accidental overdose (e.g. one injection every 24 hours instead of every 48 hours), contact your doctor or the Poisons Information Centre (In Australia telephone 131 126, in New Zealand telephone 0800 764 766) for recommendations on the management and treatment of overdosage.
While you are using Betaferon
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Betaferon.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
If you become pregnant while taking this medicine, tell your doctor immediately.
Keep all of your doctor’s appointments so that your progress can be checked. Your doctor may also take blood tests prior to commencing and regularly during treatment with Betaferon to test for changes in your blood count, liver function and thyroid function.
Tell your doctor immediately if you:
- Experience depression or suicidal thoughts;
- Experience symptoms such as itching, swelling of the face and/or your tongue or sudden shortness of breath. These may be symptoms of a serious allergic reaction (hypersensitivity) that may become life-threatening;
- Experience irregularity of your heartbeats or fluid retention (swelling) in the lower parts of your body (e.g. ankles, legs) or shortness of breath. These may be symptoms of a disease of the heart muscle which has been reported in rare cases during treatment with Betaferon.
Tell your doctor if you:
- Suffer from signs of frequent infections such as fever or a sore throat; notice unusual bruising or excessive bleeding after injury;
- Notice a yellowish colouration of your skin or eyes (jaundice), loss of appetite, or fatigue.
Injection site skin breakdown and tissue destruction can be extensive and may involve scar formation. If you have multiple lesions, Betaferon must be discontinued until healing has taken place. Patients with single lesions may continue on Betaferon provided the necrosis is not too extensive, as some patients have experienced healing of injection site necrosis while on Betaferon.
To minimise the risk of injection site necrosis you must:
- Use an aseptic technique;
- Rotate the injection sites with each dose.
The procedure of self-administration must be reviewed periodically by your doctor, especially if injection site reactions have occurred.
Things you must not do
Do not use Betaferon to treat any other complaints.
Do not stop using Betaferon, or change the dose, without first checking with your doctor.
Do not give this medicine to anyone else, even if their symptoms seem similar to yours or if they have the same condition as you.
Things to be careful of
The effects of the disease or of Betaferon treatment may influence your ability to drive a car or operate machinery. You should discuss this with your doctor if you are concerned.
After using Betaferon
Storage
Keep Betaferon in the original pack until it is time for it to be used/given.
Keep Betaferon in a cool dry place where the temperature stays below 25°C before reconstitution. Do not freeze.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
After preparing the solution, you should use it immediately. However, if you are not able to do so, you can store the reconstituted solution in the refrigerator between 2 to 8°C for up to 3 hours. (Refrigerate. Do not freeze).
When you use the solution, warm it up in your hands before injecting to avoid pain. Do not use Betaferon if you notice it contains particles or if it is discoloured.
Once you have administered the injection, you should throw any unused portion away.
Disposal
Betaferon should be used once only. After injecting, you should discard the syringe even if you have not injected all its contents. Syringes should be discarded in an appropriate disposal unit.
If your doctor tells you to stop using Betaferon or if it has passed its expiry date, ask your doctor or pharmacist what to do with any syringes that are left over.
Further information
You can obtain more information from your doctor, pharmacist or the MS Society in your State:
ACT Freecall – 1800 356 354
NSW Freecall – 1800 287 367
QLD Freecall – 1800 177 591
SA Freecall – 1800 812 311
NT Freecall – 1800 812 311
TAS Freecall – 1800 6220 1111
VIC Freecall – 1800 287 367
WA – 08 9365 4888
Schedule of Betaferon
Betaferon is an S4, prescription only medicine.
Side effects of Betaferon
Tell your doctor as soon as possible if you do not feel well while you are taking Betaferon. Betaferon helps most people with MS, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
At the beginning of treatment, side effects are common but in general, they become less with further treatment.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you.
This list contains the more common side effects of your medicine:
- Injection site reactions including redness, swelling bruising, pain or itching. These may be reduced by administration with an autoinjector. Please consult your doctor for further information;
- Flu-like symptoms including fever, chills, muscular pain, headache, tiredness, painful joints, general feeling of being unwell, or sweating. These may be relieved if paracetamol or ibuprofen is taken when the injection is given.
This list contains the other common side effects of your medicine:
- Redness, swelling, discolouration, pain, allergy, skin cell-death where the injection was given. These tend to be worst at the start of treatment and become less of a problem over time. If you experience multiple skin sores, very severe sores or breakage of the skin associated with swelling or drainage of fluid from the injection site you should discuss this with your doctor. It may be necessary to stop using Betaferon until these are healed;
- Rash, itchiness, urticaria (hives), skin discolouration, sweating;
- Nausea, vomiting, diarrhoea, constipation, abdominal pain;
- Dizziness, anxiety, nervousness;
- Infected sinus;
- Conjunctivitis.
If any of the side effects get serious, tell your doctor or pharmacist.
The list below includes the less common side effects of your medicine:
- Migraine;
- Palpitations;
- High blood pressure;
- Menstrual period upsets;
- Depression, emotional instability, convulsions (fits), suicide attempts, and confusion;
- Blood disorders;
- Muscle pain and stiffness;
- Muscle weakness;
- Shortness of breath, wheeze;
- Chest pain;
- Pelvic pain;
- Abnormal vision;
- Back pain;
- Fast heartbeat;
- Kidney problems.
The following list includes the very rare side effects of your medicine:
- Disease of the heart muscle;
- When Betaferon has been given to patients who have a very rare disease where abnormal proteins are found in the blood there have been problems with small blood vessels leading to collapse and even death;
- Loss of scalp hair;
- Serious allergic reactions;
- Disturbances of the thyroid gland;
- Hepatitis, liver failure;
- Cystitis;
- Breast pain;
- Joint pain;
- Menorrhagia (heavy menstruation);
- Infection;
- Insomnia;
- Urinary disorders;
- Anorexia;
- Convulsion;
- Changes in weight.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- Sudden signs of allergy such as rash, itching or hives on the skin, swelling of the face, lips, tongue or other parts of the body;
- Shortness of breath, wheezing or trouble breathing;
- Yellowing of the skin and/or eyes (jaundice).
These are rare but very serious side effects. You may need urgent medical attention or hospitalisation.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell. Other side effects not listed above may also occur in some people.
For further information talk to your doctor.
References
Dates
Tags
Created by:

 Login
Login













