New knowledge heralds improved treatment for metastatic renal cell carcinoma
Renal cell carcinoma (RCC) affects 270,000 people per annum worldwide and its incidence is on the rise.1 Metastatic disease in this setting has traditionally been associated with a poor prognosis2 due to resistance to conventional treatment in the form of chemotherapy and immunotherapy.3,4 Recent elucidation of the molecular pathways involved in renal carcinogenesis has opened the gateway to the development of novel treatment agents which are already yielding promising results.5
Renal cell carcinomas background
Renal cell carcinomas (RCC) arise from epithelial cells6 within the nephrons of the kidney.7 RCC can be further classified according to histological subtypes, including clear cell renal carcinomas, papillary renal carcinomas, chromophobe tumours, oncocytomas and collecting duct tumours.6,8 Clear cell RCCs are the most common subtype and account for 75–80% of RCCs.7
Most forms of RCC occur sporadically, with less than 4% of renal tumours being attributable to hereditary syndromes. The most common types of inherited kidney epithelial tumours are von Hippel–Lindau (VHL) clear cell RCC, hereditary papillary RCC, hereditary leiomyomatosis RCC and Birt–Hogg–Dubé syndrome. These inherited forms of RCC tend to present at any earlier age and are associated with multiple and often bilateral tumours.9
Renal cell carcinoma is classically associated with the triad of flank pain, haematuria and a palpable abdominal mass. Approximately 50% of RCCs are now detected, however, as incidental findings on radiographic studies.10 These tumours are more likely to be localised and of a lower stage and can therefore present without clinical signs.7 It is estimated that one quarter of RCCs are locally invasive or metastatic at the time of diagnosis. Prognosis is strongly linked to disease staging, with stage I disease having a 5-year survival rate of 95% and stage IV disease having a 5-year survival rate of 20%.10 Metastatic RCC (mRCC) carries a particularly poor prognosis.2
 | For more information about the structure and function of the kidneys, see Anatomy of the Renal System. |
 | For more information about RCC, including risk factors and treatments, see Renal Cell Carcinoma (RCC). |
Kidney cancer on the rise
Kidney cancer was the 9th most common cancer in Australia in 2005, affecting 2,297/100,000 persons. In this same year, cancer of the kidney was responsible for 852 deaths/100,000 persons.11 On a global scale, 270,000 cases of renal cancer were reported worldwide in 2008 and accounted for 116,000 deaths.6
The incidence of renal cell carcinoma is on the rise, with projections showing 67 more new cases/100,000 being diagnosed each year between 2006 and 2010 in Australia.11 One study showed that the incidence of renal cell carcinoma in North America increased by 2.39% from 1988 to 2006, a rise which was suggested to be attributable to increased detection of localised RCCs.1
RCC is more common in men than in women, occurring at a ratio between 1.5 and 2.5 to 1. The highest incidence of RCC is in the 6th and 7th decades of life.7,10 The highest rates of kidney cancer are found in North America, Europe and Australia, with Asia having one of the lowest rates.7
Kidney cancer and resistance to conventional therapy
Historically, mRCC has been associated with a poor prognosis, with Australian statistics from 2008 showing a 5-year survival rate of 6%.3 This is thought to be secondary to the ability of kidney cancers to activate pro-survival pathways and evade apoptotic pathways.4 Prior to the discovery of targeted therapy, the only treatment options available for `mRCC were chemotherapy and immunotherapy agents (including interleukin-2 and interferon alpha) which reported objective response rates of 4–6%6 and 10–22% respectively.2
According to Dr David Goldstein, Conjoint Clinical Professor at the University of New South Wales and Medical Oncologist at Prince of Wales Hospital in NSW, oncologists worked with a “long list of relatively ineffective chemotherapy options for patients with mRCC”, leading to a rather bleak outlook for patients.
The last decade has seen dramatic changes in the understanding of molecular pathways associated with renal carcinogenesis,12 largely stemming from research into the inherited syndrome of VHL and its association with RCC.8,12 This knowledge has revolutionised the management of mRCC and the development of new targeted therapeutic agents which work to disable these pathways.5
Central to the pathogenesis of renal cell carcinoma is the discovery of the VHL gene pathway, which is implicated in both hereditary and sporadic forms of RCC.6 The VHL gene is responsible for encoding VHL protein, which acts as a tumour suppressor gene. Loss of the VHL protein through mutation results in activation of hypoxia inducible factor (HIF) which in turn activates vascular endothelial growth factor (VEGF) and platelet derived growth factor (PDGF).3,6 These proteins play a crucial role in tumour growth, proliferation, angiogenesis and survival.13 Activation of a separate pathway known as the mammalian target of rapamycin (mTOR) pathway also results in HIF accumulation and similar downstream effects.6 It is thought that the rate of synthesis of HIF is regulated by mTOR.4
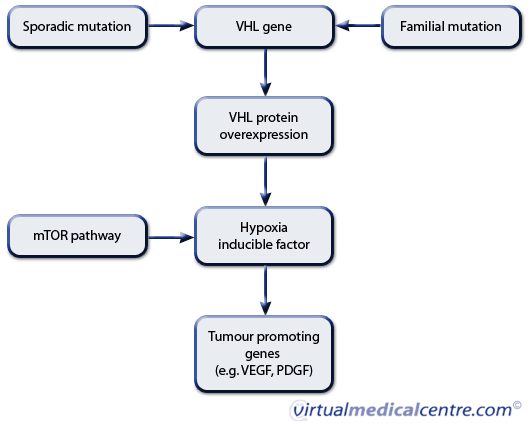
Figure 1: Molecular pathways involved in renal cell carcinogenesis
Identification of these common molecular pathways has been pivotal to the development of new agents in the battle against mRCC. A plethora of new agents which effectively inhibit these pathways12 has seen overall survival rise from an average of 10 months in 1999 to an overall survival rate in excess of 2 years in recent times.14
Dr Goldstein commented that beginning to understand the molecular pathways involved in mRCC has had a profound effect on the way this disease is viewed.
“Application of molecular biology to the management of this disease has completely altered the therapeutic landscape,” he said.
While these new therapies are palliative, not curative, Dr Goldstein says that the outlook for patients has dramatically improved.
“The goal of therapy is to prolong patient life and since the advent of biologically targeted therapies the median progression-free and overall survival has doubled,” said Dr Goldstein.
The treatment of mRCC in 2011
Three main classes of targeted agents are now available and underpin the standard of care for mRCC. These include immunotherapy, VEGF pathway inhibitors and mTOR pathway inhibitors.14
Immunotherapy
Although research indicates that VEGF and mTOR targeted therapy are more successful in the treatment of mRCC, a small subset of patients still benefit from conventional immunotherapy.14
High dose10 interleukin-2 (IL-2) agents are still considered to be a reasonable first-line treatment option in patients with mRCC.14 This is due to IL-2’s ability to induce complete remission in 5–10% of patients with mRCC.6 The median duration of response has been reported as 54 months in patients with mRCC.10 However, IL-2 has significant side effects which can affect multiple organ systems and has been reported to result in mortality in a small subgroup of patients.6 Stringent patient selection is critical to make this agent cost-effective and reduce treatment related mortality.6
Interferon-alpha (IFα) has demonstrated an overall response rate of 14% in patients with mRCC. This response is generally short-lived, with median duration of response being 6 months.10 This agent is also associated with significant side effects including flu-like symptoms, depression with suicidal ideation, malaise and dry mouth.6 It is therefore used in combination with other agents in clinical trials.10
mTOR inhibitors
The mTOR pathway plays a crucial role in several core cellular functions including protein synthesis, glucose metabolism and the migration and survival of cells.15 More importantly, mTOR plays a critical role in the rate of synthesis and transcription of HIF,4 known to be an important driver of oncogenesis.15 Agents that inhibit mTOR are therefore able to block the production of HIF and its downstream activation of tumour promoting genes.4 Two of these agents, temsirolimus (Torisel) and everolimus (Afinitor) are now approved by the US Food and Drug Administration (FDA) for the treatment of patients with mRCC.14
Unlike IL-2, mTOR inhibitors appear to be effective in the treatment of non-clear cell RCC,14 with one study showing a median survival of 11.6 months in patients with non-clear cell RCC treated with temsirolimus versus 4.3 months for the immunotherapy group.14
A recent Australian study found that this class of drugs was generally well tolerated, with the most common side effects being dyslipidaemia and hyperglycaemia.3
VEGF inhibitors (anti-angiogenic therapy)
The crucial role played by VEGF in promoting tumour angiogenesis, proliferation and immune system evasion in mRCC has stimulated the development of agents which inhibit this pathway. Agents which either inhibit the ability of VEGF to bind to its receptor, or block its receptor directly, now form the backbone of systemic therapy for mRCC.16
Three agents which are classified as tyrosine kinase inhibitors, sunitinib (Sutent), sorafenib (Nexavar) and pazopanib, selectively block the VEGF receptor. Bevacizumab (Avastin) is a monoclonal antibody which inhibits the ability of VEGF to bind to its receptor. Sunitinib, pazopanib and bevacizumab are listed in the NCCN 2011 guidelines as first-line treatment of mRCC.6 Second generation agents which have greater affinity for the VEGF receptor (e.g. axitinib and tivozanib) bring hope for greater efficacy with less toxicity. The downside of treatment with these agents is that responses are neither complete nor durable off therapy.14
The most significant side effect associated with VEGF inhibition was hypertension which affected a third of patients in a recent study on targeted therapy in mRCC. Cardiac toxicity in the form of a decline in left ventricular ejection fraction was another common side effect associated with this class of drugs. This has been reported in up to one third of patients taking sunitinib or sorafenib, but appears to be reversible.3 Other common side effects associated with VEGF inhibitors included cutaneous manifestations in the form of stomatitis, mucositis, hand–foot syndrome, rash, fatigue, diarrhoea and asymptomatic proteinuria.3,16 Discontinuation of treatment due to drug toxicity ranged from 3% to 19%.16
According to Dr Goldstein, while fatigue is a common side effect, it is quite manageable.
“The single most common side effect seen with targeted therapy is fatigue although applying a regimen of 4 weeks on treatment, followed by 2 weeks off, offers some relief and improved quality of life for patients,” he said.
Current treatment recommendations for mRCC
Table 1: Treatment recommendations for mRCC6
| Prognostic risk group | First line | Second line |
|---|---|---|---|
Clear cell RCC | Favourable risk | Sunitinib, pazopanib, bevacizumab + IFN-α; IL-2 in selected patients | Failed cytokine therapy – sorefenib, sunitinib |
| Intermediate risk | Sunitinib, pazopanib, bevacizumab + IFN-α | Failed TKI therapy – everolimus |
| Poor risk | Temsirolimus, sunitinib |
|
Non-clear cell RCC | All risk groups | Temsirolimus; sunitinib | Clinical trials |
Where to from here?
The advent of targeted therapy has opened the gateway to further research into mRCC and provided exciting new therapy options. Future research is needed to examine the effect of different dosing schedules, treatment sequencing, developing biomarkers to predict treatment response and the use of combination therapy.14
Dr Goldstein supports this view and believes that the next significant leap in improved mRCC management will be made with combination therapies, and we won’t have to wait too long to benefit from the results.
“Developing therapies that target multiple molecular pathways at the same time will be the key to preventing the development of treatment-resistant cancers and we can expect significant developments in this regard in the next 5 years or so,” said Dr Goldstein.
While more work is needed, great strides have already been made toward improving patient survival. Significantly, elucidation of underlying mRCC molecular pathways has for the first time given us the ability to develop targeted therapies for this particularly difficult to treat carcinoma.
References
- Sun M, Thuret R, Abdollah F, et al. Age-adjusted incidence, mortality and survival rates for stage-specific renal cell carcinoma in North America: A trend analysis. Eur Urol. 2011;59(1):135-41. [Abstract | Full text]
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794-9. [Abstract | Full text]
- Webber K, Cooper A, Kleiven H, et al. Management of metastatic renal cell carcinoma in the era of targeted therapies. Intern Med J. 2011;41(8):594-605. [Abstract]
- Kaelin WG Jr. Treatment of kidney cancer: Insights provided by the VHL tumor-suppressor protein. Cancer. 2009;115(10 Suppl):2262-72. [Abstract | Full text]
- Chowdhury S, Matrana MR, Tsang C, et al. Systemic therapy for metastatic non-clear-cell renal cell carcinoma: recent progress and future directions. Hematol Oncol Clin North Am. 2011;25(4):853-69. [Abstract]
- Wright I, Kapoor A. Current systemic management of metastatic renal cell carcinoma: First line and second line therapy. Curr Opin Support Palliat Care. 2011;5(3):211-21. [Abstract]
- Cho E, Adami HO, Lindblad P. Epidemiology of renal cell cancer. Hematol Oncol Clin North Am. 2011;25(4):651-65. [Abstract]
- Li L, Kaelin WG Jr. New insights into the biology of renal cell carcinoma. Hematol Oncol Clin North Am. 2011;25(4):667-86. [Abstract]
- Lopez-Beltran A, Carrasco JC, Cheng L, et al. 2009 update on the classification of renal epithelial tumors in adults. Int J Urol. 2009;16(5):432-43. [Abstract | Full text]
- Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353(23):2477-90. [Abstract]
- Cancer in Australia: An overview, 2008 [online]. Canberra, ACT: Australian Institute of Health and Welfare; 19 December 2008 [cited 15 November 2011]. Available from: URL link
- Milella M, Felici A. Biology of metastatic renal cell carcinoma. J Cancer. 2011;2:369-73. [Abstract | Full text]
- Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365(6):537-47. [Abstract]
- Cho DC, Atkins MB. Future directions in renal cell carcinoma: 2011 and beyond. Hematol Oncol Clin North Am. 2011;25(4):917-35. [Abstract]
- Voss MH, Molina AM, Motzer RJ. mTOR inhibitors in advanced renal cell carcinoma. Hematol Oncol Clin North Am. 2011;25(4):835-52. [Abstract]
- Albiges L, Salem M, Rini B, Escudier B. Vascular endothelial growth factor-targeted therapies in advanced renal cell carcinoma. Hematol Oncol Clin North Am. 2011;25(4):813-33. [Abstract]
More information
 | For more information on kidney cancer, including types and treatment, see Kidney Cancer . |
Dates
Tags
Created by:

 Login
Login














