Induction therapies for proliferative lupus nephritis
Lupus nephritis (LN) is a challenging condition for nephrologists to treat. Based on the current concepts of pathogenesis, immunosuppressive therapies remain the best treatment options at initial induction, and in the longer term.
Pioneering studies conducted by the National Institute of Health (NIH) established the role of cyclophosphamide (CYC) as the standard therapy in the management of LN.1-5 The addition of either CYC or azathioprine (AZA) reduces the risk of dying or the development of end stage renal disease (ESRD) as compared to prednisolone alone.6 However, the long term use of CYC is associated with significant complications in the form of amenorrhoea, infections and malignancy.7,8
Flanc et al. have reviewed evidence for the treatment of LN.9 They suggested that CYC plus steroids reduced the risk of doubling of serum creatinine compared to steroids alone, but had no impact on mortality. In addition, the risk of ovarian failure was significantly increased. AZA plus steroids reduced the risk of all causes of mortality compared to steroids alone but did not alter renal outcomes.
Despite the lack of great success and high rate of complications, most physicians continue to use CYC as part of the treatment protocol for both induction and maintenance therapy of LN. Steroids remain as an adjuvant in both forms of therapies.
LN is predominantly seen in young patients. Therefore, preserving reproductive function is imperative. In an effort to minimise complications, the European Lupus Nephritis study group (ELNT) used a fixed dose pulse CYC for induction (500 mg pulses every 2 weeks for 6 doses).10 They reported improved complication rates and similar success rates, as compared to high dose CYC. On follow up (mean 97 months), it was observed that there was no significantly greater cumulative probability of ESRD or death in patients given a low dose CYC regimen. Although patient numbers were small in this study, three times more successful pregnancies occurred in patients who received low dose rather than high dose therapy. 11
There are several options available that need to be considered in all patients with LN if one intends to use CYC (Table 1).12
Table 1: Options for fertility preservation in CYC treated SLE patients
1. General considerations | ||||||||
2. Female patients
| ||||||||
3. Male patients
| ||||||||
| (Reprinted with kind permission from: Raptopoulou A, Sidiropoulos P, Boumpas DT. Ovarian failure and strategies for fertility preservation in patients with systemic lupus erythematosus. Lupus. 2004; 13: 887-90.) |
Due to the poor safety profile of CYC, many investigators have tried other agents, including calcineurin inhibitors, in the management of proliferative LN.13,14 Most of these studies are single centre and open labelled and report variable success rates. Relapse following cessation of therapy remains a major problem in a significant number of these patients.
A recent study has reported the outcome of patients with LN treated with cyclosporine (CsA), where CsA was used as first-line treatment in 38.7% of patients and as second-line treatment in 61.3% of patients.15 Complete remission was achieved in 93.5% of patients. The relapse rate was 45.2%. The mean disease-free interval was 33 months. At the end of follow-up, a total of 67.9% of the patients were in remission. However, the optimal dose of CsA in the treatment of LN was not well studied and long-term calcineurin toxicity was not defined either. Leflunomide has also been reported to be useful in the management of resistant LN.16 Although none of these agents should be the first line for induction therapy, they may have a role in cases of LN resistant to standard immunosuppressive therapy.
With experience gained from transplant populations, many researchers have attempted to use mycophenolate mofetil (MMF) in the management of proliferative LN.17-19 Initial studies involving MMF as maintenance therapy (after induction of remission with CYC and steroids) reported a good safety profile and sustained remission rates. Later studies used MMF as an induction agent in LN, in comparison with CYC, and showed improved remission rates and fewer side effects. 20-23 In particular, leukopenia and amenorrhoea occurred more frequently in CYC treated patients than in MMF treated patients.25
The evidence that MMF is a superior treatment option to CYC is mixed. Moore et al. published a systematic review and meta-analysis of randomised trials (RCT) and cohort studies of MMF in LN.24 They concluded that MMF produced more complete responses and complete plus partial responses than CYC. However, it was also noted that there are limitations to the existing data, not the least of which is the short-term results relative to the very long course of LN. A more recent study comparing MMF to CYC found that MMF was not superior to intravenous CYC in inducing treatment response in LN. 26
| Figure 1 plots the proportion of patients in each trial with a complete and partial response with MMF or CYC and shows the variability between individual trials. The blue circles show trials with oral agents, with the sole maintenance trial using oral agents in dark blue. The inset scale represents the overall number of patients in each comparison. |
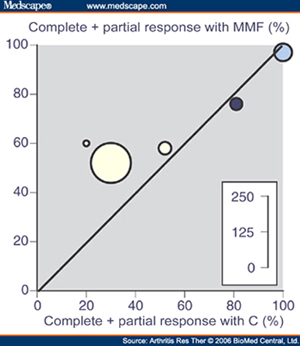
| (Reprinted with kind permission from: Moore RA, Derry S. Systematic review and meta-analysis of randomised trials and cohort studies of mycophenolate mofetil in lupus nephritis. Arthritis Res Ther. 2006; 8(6): R182.) |
Rituximab is a monoclonal antibody directed against the CD20 marker of B cells. Because of its ability to deplete B lymphocytes, it has been suggested that the drug could be of benefit in B cell-dependent diseases, including systemic lupus erythematosus (SLE). However, most of the case reports and small case series used B cell depletion in severe and/or resistant LN.
Two recent studies have investigated the histopathologic and clinical effects of combination treatment with rituximab and CYC in patients with CYC-resistant proliferative LN.27,28 At 6 month follow-up, significant clinical improvements were noted in Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) scores, anti-double-stranded DNA antibody levels, and anti-C1q antibody levels. On repeat renal biopsy, improvement in the histopathologic class of nephritis occurred in a majority of patients, and a decrease in the renal activity index was noted. In patients with proliferative LN who fail to respond to conventional immunosuppressive therapy including CYC, combined treatment with rituximab and CYC may constitute a new treatment option.
Additional complexities occur in the management of LN in patients with severe renal dysfunction. Studies with MMF typically excluded these types of patients. In a recent report, Chan et al. reported encouraging results using MMF in patients with LN with non-necrotising vasculopathy.29 A recent retrospective study compared the effects, relapse ratio and outcomes between MMF and pulse intravenous cyclophosphamide (CTX) for the induction therapy in patients with crescentic LN.30 They observed a higher complete remission ratio and lower relapse ratio in the MMF group than in the CTX group. The side effect of infection was less frequent in the MMF group, which showed preferable security of MMF.
Until this issue is settled, CYC remains the ‘standard therapy’ for this group of patients. Given below is the protocol suggested by Ponticelli for patients of LN with severe renal dysfunction.31
Figure 2 depicts the proposed management flow chart in patients with LN and severe renal involvement at presentation or at renal flares.
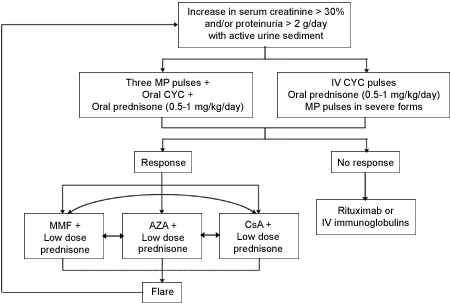
| (Reprinted with kind permission from: Ponticelli C. New therapies for lupus nephritis. Clin J Am Soc Nephrol. 2006; 1(4): 863-8.) |
As results from long-term studies with newer drugs become available, optimal therapies for the treatment and management of LN may become clearer. The results available so far from MMF studies are encouraging in patients without severe renal dysfunction and provide a possible alternative treatment to CYC therapy for LN patients.
Article written by:
Dr Sree Krishna Venuthurupalli, MD DM
Renal Fellow, Royal Brisbane & Women’s Hospital
Dr Dwarakanathan Ranganathan, MD DM FRCP FRACP
Senior Consultant Nephrologist, Royal Brisbane & Women’s Hospital
Editorial Advisory Board Member of the Virtual Neuro Centre
Acknowledgements:
We thank Dr Adrian Kark for his comments on the manuscript.
References:
- Austin HA, Klippel JH, Balow JE, le Riche NG, Steinberg AD, Plotz PH, et al. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med. 1986; 314(10): 614-9.
- Steinberg AD, Steinberg SC. Long-term preservation of renal function in patients with lupus nephritis receiving treatment that includes cyclophosphamide versus those treated with prednisone only. Arthritis Rheum. 1991; 34(8): 945-50.
- Boumpas DT, Austin HA, Vaughn EM, Klippel JH, Steinberg AD, Yarboro CH, et al. Controlled trial of pulse methylprednisolone versus two regimens of pulse cyclophosphamide in severe lupus nephritis. Lancet. 1992; 340(8822): 741-5.
- Gourley MF, Austin HA, Scott D, Yarboro CH, Vaughan EM, Muir J, et al. Methylprednisolone and cyclophosphamide, alone or in combination, in patients with lupus nephritis. A randomized, controlled trial. Ann Intern Med. 1996; 125(7): 549-57.
- Illei GG, Austin HA, Crane M, Collins L, Gourley MF, Yarboro CH, et al. Combination therapy with pulse cyclophosphamide plus pulse methylprednisolone improves long-term renal outcome without adding toxicity in patients with lupus nephritis. Ann Intern Med. 2001; 135(4): 248-57.
- Bansal VK, Beto JA. Treatment of lupus nephritis: A meta-analysis of clinical trials. Am J Kidney Dis. 1997; 29(2): 193-9.
- Boumpas DT, Austin HA, Vaughan EM, Yarboro CH, Klippel JH, Balow JE. Risk for sustained amenorrhea in patients with systemic lupus erythematosus receiving intermittent pulse cyclophosphamide therapy. Ann Intern Med. 1993; 119(5): 366-9.
- Radis CD, Kahl LE, Baker GL, Wasko MC, Cash JM, Gallatin A, et al. Effects of cyclophosphamide on the development of malignancy and on long-term survival of patients with rheumatoid arthritis. A 20-year follow-up study. Arthritis Rheum. 1995; 38(8): 1120-7.
- Flanc RS, Roberts MA, Strippoli GF, Chadban SJ, Kerr PG, Atkins RC. Treatment of diffuse proliferative lupus nephritis: A meta-analysis of randomized controlled trials. Am J Kidney Dis. 2004; 43(2): 197-208.
- Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani GD, Garrido Ed Ede R, Danieli MG, et al. Immunosuppressive therapy in lupus nephritis: The Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002; 46(8): 2121-31.
- Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani GD, Garrido Ed Ede R, Danieli MG, et al. Long-term outcome of patients randomized in the Euro-Lupus Nephritis Trial: Further evidence that a low dose IV cyclophosphamide induction regimen achieves good results. Ann Rheum Dis. 2006; 65 (Suppl 2): 64.
- Raptopoulou A, Sidiropoulos P, Boumpas DT. Ovarian failure and strategies for fertility preservation in patients with systemic lupus erythematosus. Lupus. 2004; 13(12): 887-90.
- Mok CC, Tong KH, To CH, Siu YP, Au TC. Tacrolimus for induction therapy of diffuse proliferative lupus nephritis: An open-labeled pilot study. Kidney Int. 2005; 68(2): 813-7.
- Tam LS, Li EK, Leung CB, Wong KC, Lai FM, Wang A, et al. Long-term treatment of lupus nephritis with cyclosporine A. QJM. 1998; 91(8): 573-80.
- Rihova Z, Vankova Z, Maixnerova D, Dostal C, Jancova E, Honsova E, et al. Treatment of lupus nephritis with cyclosporine – An outcome analysis. Kidney Blood Press Res. 2007; 30(2): 124-8.
- Tam LS, Li EK, Wong CK, Lam CW, Li WC, Szeto CC. Safety and efficacy of leflunomide in the treatment of lupus nephritis refractory or intolerant to traditional immunosuppressive therapy: An open label trial. Ann Rheum Dis. 2006; 65(3): 417-8.
- Dooley MA, Cosio FG, Nachman PH, Falkenhain ME, Hogan SL, Falk RJ, et al. Mycophenolate mofetil therapy in lupus nephritis: Clinical observations. J Am Soc Nephrol. 1999; 10(4): 833-9.
- Kingdon EJ, McLean AG, Psimenou E, Davenport A, Powis SH, Sweny P, et al. The safety and efficacy of MMF in lupus nephritis: A pilot study. Lupus. 2001; 10(9): 606-11.
- Kapitsinou PP, Boletis JN, Skopouli FN, Boki KA, Moutsopoulos HM. Lupus nephritis: Treatment with mycophenolate mofetil. Rheumatology (Oxford). 2004; 43(3): 377-80.
- Chan TM, Li FK, Tang CS, Wong RW, Fang GX, Ji YL, et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong-Guangzhou Nephrology Study Group. N Engl J Med. 2000; 343(16): 1156-62.
- Ginzler E, Aranow C, Buyon J, Dooley MA, Merrill JT, Petri M, et al. A multicenter study of mycophenolate mofetil (MMF) vs. intravenous cyclophosphamide (IVC) as induction therapy for severe lupus nephritis (LN): Preliminary results. Arthritis Rheum. 2003; 48(Suppl): S647.
- Chan TM, Tse KC, Tang CS, Mok MY, Li FK. Long-term study of mycophenolate mofetil as continuous induction and maintenance treatment for diffuse proliferative lupus nephritis. J Am Soc Nephrol. 2005; 16(4): 1076-84.
- Ginzler EM, Dooley MA, Aranow C, Kim MY, Buyon J, Merrill JT, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005; 353(21): 2219-28.
- Moore RA, Derry S. Systematic review and meta-analysis of randomised trials and cohort studies of mycophenolate mofetil in lupus nephritis. Arthritis Res Ther. 2006; 8(6): R182.
- Walsh M, James M, Jayne D, Tonelli M, Manns BJ, Hemmelgarn BR. Mycophenolate mofetil for induction therapy of lupus nephritis: A systematic review and meta-analysis. Clin J Am Soc Nephrol. 2007; 2(5): 968-75.
- Sinclair A, Appel G, Dooley MA, Ginzler E, Isenberg D, Jayne D, et al. Protocol for the Aspreva Lupus Management Study (ALMS). Lupus. 2007; 16(12): 972-80.
- Gunnarsson I, Sundelin B, Jónsdóttir T, Jacobson SH, Henriksson EW, van Vollenhoven RF. Histopathologic and clinical outcome of rituximab treatment in patients with cyclophosphamide-resistant proliferative lupus nephritis. Arthritis Rheum. 2007; 56(4): 1263-72.
- Jónsdóttir T, Gunnarsson I, Risselada A, Henriksson EW, Klareskog L, van Vollenhoven RF. Treatment of refractory SLE with rituximab plus cyclophosphamide: Clinical effects, serological changes, and predictors of response. Ann Rheum Dis. 2008; 67(3): 330-4.
- Wang J, Hu W, Xie H, Zhang H, Chen H, Zeng C, et al. Induction therapies for class IV lupus nephritis with non-inflammatory necrotizing vasculopathy: Mycophenolate mofetil or intravenous cyclophosphamide. Lupus. 2007; 16(9): 707-12.
- Tang Z, Yang G, Yu C, Yu Y, Wang J, Hu W, et al. Effects of mycophenolate mofetil for patients with crescentic lupus nephritis. Nephrology (Carlton). 2008 Sep 1. [Epub ahead of print]
- Ponticelli C. New therapies for lupus nephritis. Clin J Am Soc Nephrol. 2006; 1(4): 863-8.
Dates
Tags
Created by:

 Login
Login














